Abstract
Background: Both the anti-CD22 antibody-drug conjugate inotuzumab ozogamicin (INO) and the CD3-CD19 bispecific T-cell engager blinatumomab have single-agent activity in relapsed or refractory acute lymphoblastic leukemia (ALL). We previously reported the promising efficacy and survival of INO in combination with low-intensity mini-hyper-CVD chemotherapy in older adults with newly diagnosed ALL (Kantarjian H et al, Lancet Oncol 2018;19(2):240-8). We sought to improve these outcomes by adding blinatumomab to this regimen.
Methods: Patients (pts) ≥60 years of age with newly diagnosed Philadelphia chromosome-negative pre-B ALL were eligible. Pts were required to have a performance status of ≤3, total bilirubin ≤1.5 mg/dl, AST/ALT ≤3x ULN and creatinine ≤2 mg/dl. Pts received mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses) for up to 8 cycles. INO was given at a dose of 1.3-1.8mg/m2 on day 3 of cycle 1 and 0.8-1.3mg/m2 on day 3 of cycles 2-4. Pts 1-6 received 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; pts 7+ received the phase II dose of 1.8 mg/m2 for cycle 1 followed by 1.3 mg/m2 for subsequent cycles. Rituximab (if CD20+) and prophylactic IT chemotherapy were given for the first 4 cycles. Responding pts received POMP maintenance for up to 3 years. After the observation of veno-occlusive disease (VOD), the protocol was amended in 9/2015 to use lower doses of INO. After this amendment (pts 35+), INO was given at 1.3 mg/m2 for cycle 1 and 1 mg/m2 for subsequent cycles. Another amendment was made in 3/2017 (pts 50+) to give INO in split doses each cycle (0.6 mg/m2 on day 2 and 0.3 mg/m2 on day 8 of cycle 1; 0.3 mg/m2 on day 2 and 8 of cycles 2-4) and 4 cycles of blinatumomab at standard dosing after the INO-based cycles, for a total of 4 cycles, and before the initiation of the maintenance therapy (i.e. cycles 5-8).
Results: 58 pts have been treated, 4 of whom were in complete remission (CR) at enrollment. Median age was 68 years (range, 60-81 years) and median CD22 expression was 97% (range, 27-100%). 31 pts (53%) were CD20+ and received rituximab.
Among 54 pts evaluable for morphologic response, 53 (98%) responded (CR, n=47; CRp, n=5; CRi, n=1). Only 1 pt did not respond. MRD negativity by 6-color flow cytometry was achieved in 39/52 pts (75%) after 1 cycle and 54/57 pts (95%) overall. There were no early deaths, and the 30-day and 60-day mortality rates were 0% and 3%, respectively.
Among 57 who achieved remission, 8 (14%) relapsed, 3 (5%) underwent allogeneic SCT in CR1, 31 (54%) remain on treatment or have completed maintenance, and 17 (30%) died in CR/CRp. Causes of death for pts in CR/CRp included: sepsis (n=8), VOD (n=3), gunshot wound (n=1), dementia and deconditioning (n=1), end stage renal disease (n=1) and unknown causes (n=3). 5 pts (8%) developed VOD, 1 after subsequent allogeneic SCT. The rate of VOD was 4/49 (8%) prior to amendment #2 and 1/9 (11%) after the amendment.
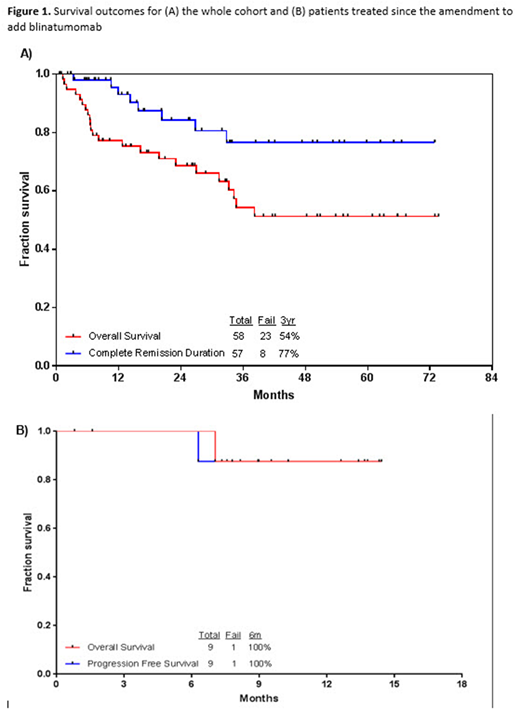
With a median follow-up of 28 months (range, 2-68 months), 35 pts (60%) were alive, 32 of whom (55%) were in CR and MRD negative status. The 3-year continued remission and OS rates were 77% and 54%, respectively (Figure 1A). The outcomes of the 9 pts treated since amendment #2 are shown in Figure 1B. One pt developed VOD and died after 4 cycles of mini-hyper-CVD. Compared to a similar historical cohort of older pts treated with hyper-CVAD ± rituximab (n=77), mini-hyper-CVD + INO ± blinatumomab resulted in significantly higher 3-year OS (54% vs 32%; P=0.002).
Conclusion: Mini-hyper-CVD + INO ± blinatumomab, is safe and effective in elderly pts with newly diagnosed Ph-negative ALL, with an overall response rate of 98% and 3-year OS rate of 54%.
Short:Takeda Oncology: Consultancy. Jabbour:Abbvie: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Ravandi:Amgen: Honoraria, Research Funding, Speakers Bureau; Sunesis: Honoraria; Jazz: Honoraria; Xencor: Research Funding; Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Sunesis: Honoraria; Abbvie: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Seattle Genetics: Research Funding; Macrogenix: Honoraria, Research Funding; Jazz: Honoraria; Xencor: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Orsenix: Honoraria. Jain:Pfizer: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Infinity: Research Funding; Cellectis: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Genentech: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; ADC Therapeutics: Research Funding; Cellectis: Research Funding; Pfizer: Research Funding; Verastem: Research Funding; BMS: Research Funding; Servier: Research Funding; Astra Zeneca: Research Funding; Infinity: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Research Funding; Pharmacyclics: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sasaki:Otsuka Pharmaceutical: Honoraria. Pemmaraju:novartis: Research Funding; cellectis: Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; stemline: Consultancy, Honoraria, Research Funding; plexxikon: Research Funding; daiichi sankyo: Research Funding; abbvie: Research Funding; Affymetrix: Research Funding; SagerStrong Foundation: Research Funding. Daver:Pfizer: Consultancy; Otsuka: Consultancy; Daiichi-Sankyo: Research Funding; Novartis: Research Funding; ImmunoGen: Consultancy; Sunesis: Consultancy; Incyte: Consultancy; Sunesis: Research Funding; Karyopharm: Consultancy; Karyopharm: Research Funding; Alexion: Consultancy; BMS: Research Funding; ARIAD: Research Funding; Kiromic: Research Funding; Incyte: Research Funding; Novartis: Consultancy; Pfizer: Research Funding. Khoury:Stemline Therapeutics: Research Funding. Konopleva:Stemline Therapeutics: Research Funding. Kadia:Takeda: Consultancy; Jazz: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Research Funding; Jazz: Consultancy, Research Funding; Abbvie: Consultancy; BMS: Research Funding; Celgene: Research Funding; Abbvie: Consultancy; Novartis: Consultancy; BMS: Research Funding; Takeda: Consultancy; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Wierda:Genentech: Research Funding; AbbVie, Inc: Research Funding. DiNardo:Abbvie: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria; Agios: Consultancy; Bayer: Honoraria; Medimmune: Honoraria. O'Brien:Vaniam Group LLC: Consultancy; Amgen: Consultancy; Gilead: Consultancy, Research Funding; Alexion: Consultancy; Abbvie: Consultancy; GlaxoSmithKline: Consultancy; Sunesis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Astellas: Consultancy; Aptose Biosciences Inc.: Consultancy; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; TG Therapeutics: Consultancy, Research Funding; Acerta: Research Funding; Celgene: Consultancy; Regeneron: Research Funding; Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.